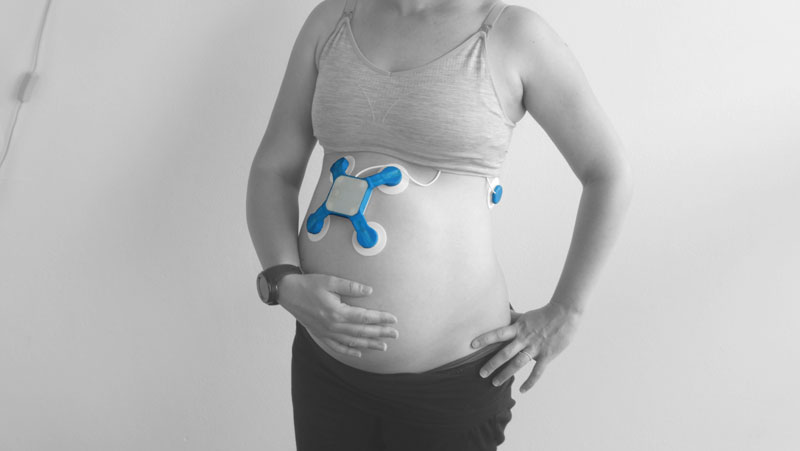
 BaymatobTM, a MedTech company founded by a Sydney mother after her own traumatic birth experience, has developed a world-first labour monitoring device that has achieved US Food and Drug Administration (FDA) Breakthrough Device Designation. This sets Baymatob’s game changing maternal diagnostic tool called OliTM on an streamlined and supported path toward regulatory approval.
BaymatobTM, a MedTech company founded by a Sydney mother after her own traumatic birth experience, has developed a world-first labour monitoring device that has achieved US Food and Drug Administration (FDA) Breakthrough Device Designation. This sets Baymatob’s game changing maternal diagnostic tool called OliTM on an streamlined and supported path toward regulatory approval.
Oli seeks to identify an individual, during labour, who is at higher risk of developing abnormal postpartum uterine bleeding or postpartum haemorrhage, in advance of delivery/birth. Globally, postpartum haemorrhage is the leading cause of preventable maternal death, with a mother dying every 7 minutes [1]. Between 5 – 15% of all mothers experience some level of postpartum haemorrhage in Australia and NZ [2].
In order to award a medical device Breakthrough Device Designation, the US Food and Drug Administration (FDA) recognises its potential to provide more effective treatment or diagnosis for a life-threatening or irreversibly debilitating disease or condition. This allows Baymatob to benefit from a streamlined and supported regulatory process on its path to commercialisation of Oli.
The FDA’s Breakthrough program aims to provide patients and health care providers with timely access to selected medical devices by speeding up development, assessment, and review. Breakthrough device designation does not signal FDA clearance. In granting Oli designation as a Breakthrough Device, the FDA recognises that Oli has the potential to save lives and reduce irreversible harm, such as from unplanned hysterectomies, that can be experienced by women as a result of postpartum haemorrhage.
Oli is intended to do what no other device on the market does – identify patients well before they give birth who are at higher risk of developing abnormal postpartum uterine bleeding or postpartum haemorrhage.
For further information please contact:
Sarah McDonald, CEO and Founder (
Tara Croft, COO (
[1] J. L. Bienstock, A. C. Eke, and N. A. Hueppchen, "Postpartum Hemorrhage," N Engl J Med, vol. 384, pp. 1635-1645, Apr 29 2021.
[2] RANZCOG, "Management of Postpartum Haemorrhage (PPH) " Royal Australian and New Zealand College of Obstetricians and GynaecologistsJuly 2017 2017.
